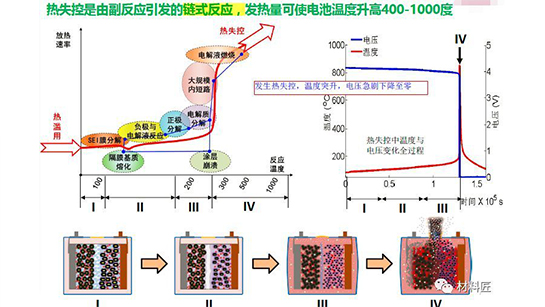
Battery thermal runaway is caused by the fact that the heat generation rate of the battery is much higher than the heat dissipation rate, and a large amount of heat accumulates but is not dissipated in a timely manner. Essentially, "thermal runaway" is an energy positive feedback cycle process: an increase in temperature causes the system to heat up, and when the system heats up, the temperature rises, which in turn makes the system hotter. Without strict division, battery thermal runaway can be divided into three stages:
Study on the kinetic mechanism of thermal runaway reaction in different types of lithium batteries
Stage 1: Internal thermal runaway stage of the battery
Due to internal short circuits, external heating, or the battery's own heating during high current charging and discharging, the internal temperature of the battery rises to around 90 íŠ to 100 íŠ, and the lithium salt LiPF6 begins to decompose; The chemical activity of the carbon negative electrode in the charging state is very high, close to metallic lithium. At high temperatures, the SEI film on the surface decomposes, and the lithium ions embedded in graphite react with the electrolyte and binder, further pushing the battery temperature to 150 íŠ. At this temperature, new intense exothermic reactions occur, such as a large amount of electrolyte decomposition, generating PF5, which further catalyzes the decomposition reaction of organic solvents, etc.
Stage 2: Battery Drum Stage
When the battery temperature reaches above 200 íŠ, the positive electrode material decomposes, releasing a large amount of heat and gas, continuously heating up. The lithium embedded negative electrode begins to react with the electrolyte at 250-350 íŠ.
Stage 3: Battery thermal runaway and explosion failure stage
During the reaction process, the charged positive electrode material begins to undergo a violent decomposition reaction, and the electrolyte undergoes a violent oxidation reaction, releasing a large amount of heat, producing high temperature and a large amount of gas, causing the battery to burn and explode.
Safety of lithium-ion battery materials
Negative electrode material
Although the negative electrode material is relatively stable, the carbon negative electrode in the lithium embedded state will react with the electrolyte at high temperatures. The reaction between the negative electrode and the electrolyte includes the following three parts: the decomposition of SEI; The reaction between lithium embedded in the negative electrode and the electrolyte; The reaction between lithium embedded in the negative electrode and the binder. The SEI film with electronic insulation at room temperature can prevent further decomposition reactions of the electrolyte. But around 100 íŠ, the decomposition reaction of SEI membrane will occur. The reaction formula for SEI exothermic decomposition reaction is as follows:
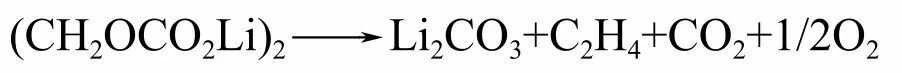
Although the heat of SEI decomposition reaction is relatively small, its initial reaction temperature is lower, which will to some extent increase the "combustion" diffusion rate of the negative electrode.
Temperature range and reaction enthalpy of various exothermic reactions in lithium-ion batteries
At higher temperatures, the negative electrode surface loses the protection of the SEI film, and the lithium embedded in the negative electrode will directly react with the electrolyte solvent to produce C2H4O, which may be acetaldehyde or ethylene oxide. The graphite embedded with lithium undergoes the following reaction with the molten PVDF-HPF copolymer at temperatures above 300 íŠ:
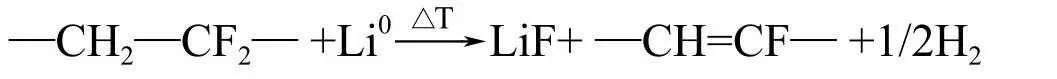
The reaction heat increases with the degree of lithium insertion, and varies with the type of binder. Increase its thermal stability through film forming additives or lithium salts. The ways to reduce the reaction heat between lithium embedded in the negative electrode and electrolyte include two aspects: reducing the lithium embedded in the negative electrode and reducing the specific surface area of the negative electrode. Reducing the amount of lithium embedded in the negative electrode means that the ratio of positive and negative electrodes must be appropriate, with an excess of about 3% to 8% for the negative electrode. Reducing the specific surface area of the negative electrode can also effectively improve the safety of the battery. According to literature reports, when the specific surface area of carbon negative electrode materials increases from 0.4m2 · g-1 to 9.2m2 · g-1, the reaction rate increases by two orders of magnitude‘
But if the surface area is too low, it will reduce the rate performance and low-temperature performance of the battery. This requires a reasonable design of the negative electrode structure and optimization of the electrolyte formula to improve the solid-state diffusion rate of lithium ions in the negative electrode and obtain SEI films with good ion conductivity. Furthermore, although the weight ratio of the binder in the negative electrode is very small, its reaction heat with the electrolyte is considerable. Therefore, reducing the amount of binder or selecting appropriate binders will be beneficial for improving the safety performance of batteries.
Through the analysis of patents, the literature also believes that the main methods to solve the safety of carbon negative electrode materials are to reduce the specific surface area of negative electrode materials and improve the thermal stability of SEI films. Technologies related to improving negative electrode materials and structures to enhance battery safety performance in existing domestic patent applications.