Lithium ion batteries have many advantages, such as high working voltage (three times that of nickel hydrogen and nickel cadmium batteries), high specific energy (up to 165Wh/kg, three times that of nickel hydrogen batteries), small size, light weight, long cycle life, low self discharge, no memory effect, and no pollution. Lithium iron phosphate batteries are highly regarded in the new energy industry, with a cycle life of around 3000 times and stable discharge. They are widely used in power batteries and energy storage fields. However, the speed of its promotion and the breadth and depth of its application fields are not as satisfactory. In addition to factors such as price and batch consistency caused by the battery material itself, the temperature performance is also an important factor that hinders its rapid promotion. Yunsheng Lithium Battery investigated the effect of temperature on the performance of lithium iron phosphate batteries, and also investigated the charging and discharging of battery packs under high and low temperature conditions. Below, Yunsheng Electronics will discuss with you the impact of temperature on lithium iron phosphate batteries.
1¡¢ Summary of room temperature cycling for individual units (modules)
The cycle life of the battery tested at room temperature shows that lithium iron phosphate battery has the advantage of long service life. Currently, it has achieved 3314 cycles, and the capacity retention rate is still at 90%. However, reaching 80% of the battery life may require about 4000 cycles of termination.
1. Monomer cycle
Currently completed: 3314 cycles, with a capacity retention rate of 90%.
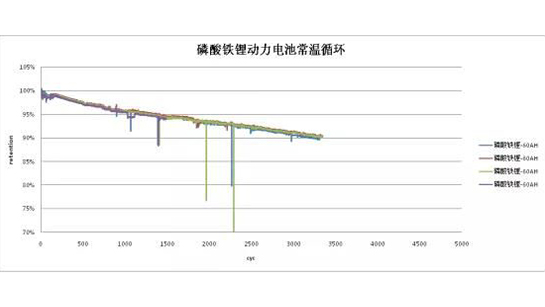
Due to the processing technology of battery cells and the grouping process of modules, inconsistencies have formed in the battery after PACKing. The more exquisite the process, the smaller the internal resistance of grouping, and the smaller the differences between battery cells. The cycle life of the following modules is currently the basic data that most lithium iron phosphate batteries can achieve. Therefore, during use, BMS needs to regularly balance the battery pack, reduce inter cell differences, and extend its service life.
2. Module loop
Currently completed: 2834 cycles, with a capacity retention rate of 67.26%.
2¡¢ Summary of high-temperature cycling of individual units
Accelerate the aging life of batteries under high temperature conditions.
1. Individual charge discharge curve
2. High temperature cycle
The high-temperature cycle completed 1100 cycles with a capacity retention rate of 73.8%.
3¡¢ The effect of low temperature on charging and discharging performance
At temperatures ranging from 0 to -20 ¡æ, the discharge capacity of the battery is equivalent to 88.05%, 65.52%, and 38.88% of the capacitance at a temperature of 25 ¡æ, respectively; The average discharge voltage is 3.134 V, 2.963 V, and 2.788 V, respectively. The average discharge voltage at 20 ¡æ is 0.431 V lower than at 25 ¡æ. From the above analysis, it can be seen that as the temperature decreases, the average discharge voltage and discharge capacity of lithium-ion batteries both decrease, especially when the temperature is -20 ¡æ, the discharge capacity and average discharge voltage of the battery decrease rapidly.
From an electrochemical perspective, the solution resistance and SEI film resistance do not change much throughout the entire temperature range, and their impact on the low-temperature performance of the battery is relatively small; The charge transfer resistance significantly increases with the decrease of temperature, and the temperature variation throughout the entire temperature range is significantly greater than the solution resistance and SEI film resistance. This is because as the temperature decreases, the ion conductivity of the electrolyte decreases, and the SEI film resistance and electrochemical reaction resistance increase, resulting in increased Ohmic polarization, concentration polarization, and electrochemical polarization at low temperatures. On the discharge curve of the battery, the average voltage and discharge capacity both decrease with decreasing temperature.
From Figure 2, it can be seen that cycling 5 times at -20 ¡æ and then cycling at 25 ¡æ results in a decrease in both the battery capacity and discharge platform. This is because as the temperature decreases, the ion conductivity of the electrolyte decreases, and the ohmic polarization, concentration polarization, and electrochemical polarization increase during low-temperature charging, leading to the deposition of metallic lithium and the decomposition of the electrolyte. Ultimately, this results in the thickening of the SEI film on the electrode surface, an increase in SEI film resistance, and a decrease in discharge platform and discharge capacity on the discharge curve.
1. The impact of low temperature on cycling performance
The capacity of the battery decays rapidly in an environment of -10 ¡æ, with only 59mAh/g remaining after 100 cycles, resulting in a capacity decay of 47.8%; The battery that has been discharged at low temperature will be tested for charge and discharge at room temperature to assess its capacity recovery performance. Its capacity has been restored to 70.8mAh/g, with a capacity loss of 68%. It can be seen that the low-temperature cycle of the battery has a significant impact on the recovery of battery capacity.
2. The impact of low temperature on safety performance
Lithium ion battery charging is the process in which lithium ions are removed from the positive electrode and migrated into the negative electrode material through electrolyte migration. The lithium ions polymerize towards the negative electrode and are captured by six carbon atoms. At low temperatures, the chemical reaction activity decreases, and the migration of lithium ions slows down. The lithium ions on the negative electrode surface have not yet been embedded in the negative electrode and have been reduced to metallic lithium, which precipitates and forms lithium dendrites on the negative electrode surface. This can easily puncture the separator and cause short circuits inside the battery, thereby damaging the battery and causing safety accidents.
From the above data, it can be concluded that lithium iron phosphate batteries are greatly affected by temperature. In the field of power battery applications and environments where temperature has a significant impact, thermal management (air cooling, liquid cooling, etc.) is required to improve battery efficiency and extend battery system lifespan.
Previous£ºIt's already the last one£¡
Next:Analyzing the common problems of lithium iron phosphate materials in battery processing